A Decade of Collaborative
Drug Discovery




A Decade of Collaborative Drug Discovery
After 11 years of intense collaboration, the European Lead Factory, a flagship pan-European early drug discovery project, has come to a close. In this final project update, we look back at the results and impacts of the decade-long collaboration.
Background
The European Lead Factory (ELF) was first established in 2013 when seven large pharmaceutical companies came together to discuss the pre-competitive sharing of their compound collections with each other and the European scientific community. The infrastructure to allow for compound sharing, screening, and the management of such an endeavour was provided by a handful of specialised Contract Research Organizations (CROs), academic partners, and a foundation.
Over time, the industry collection was supplemented with novel compounds, designed and synthesised by medicinal chemists. The ultimate goal of the ELF was to give scientists working on innovative targets and searching for drug discovery starting points access to an industry-standard screening infrastructure and allow them to screen their drug targets and phenotypes of interest against one large compound library composed of unique high-quality compounds with drug-like properties. Funded by the Innovative Medicines Initiative (IMI, now the Innovative Health Initiative), the ELF was born as a shared platform for open innovation in drug discovery – a first of its kind in Europe.
Running from 2013-2023, through the support of two consecutive grants from the IMI, the publicly co-funded project was the largest collective drug discovery platform in Europe. It has been a true example of successful public-private collaboration, bringing together the best of industry and academia to drive the development of new medical solutions for patients in need.

“The ELF has addressed the drug development needs of a wide variety of therapeutic areas. All major disease areas are covered in the ELF portfolio. Over a quarter includes infectious diseases, of which 10% are neglected diseases.”
Dr Ton Rijnders,
Chair ELF Strategic Portfolio Management Team & Scientific Leader at Lygature

Objectives
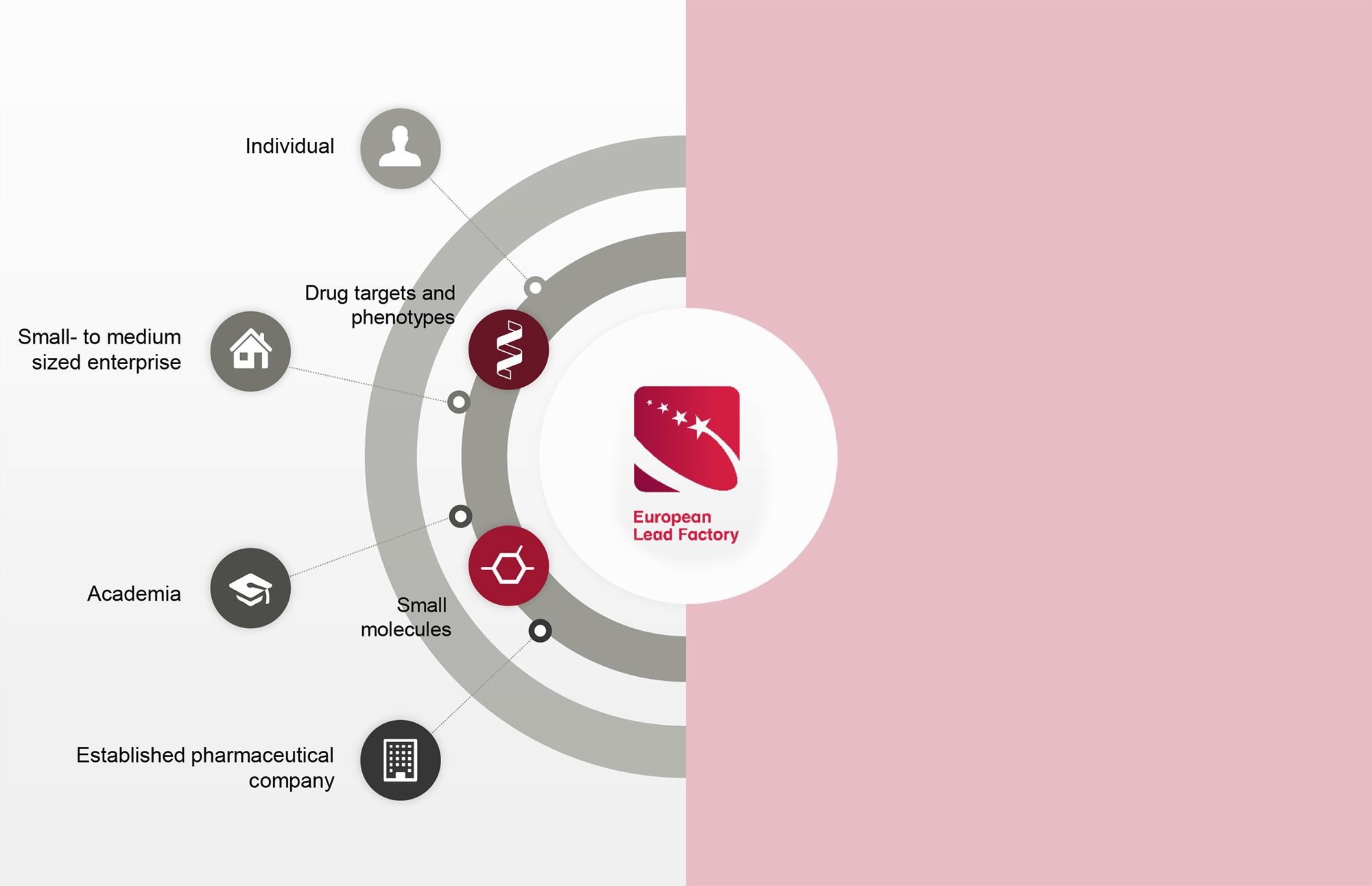
The ELF sought to jump-start early drug discovery by bridging the gap between innovative biology and creative medicinal chemistry, bringing different expertise together. To achieve this goal, the ELF established a shared platform for collaborative drug discovery. The overall objectives included:
- Creating an industry-standard screening collection of high-quality compounds with drug-like properties
- Making this library of compounds accessible to European academics and biotech SMEs via a transparent and diligent review process
- Providing access to state-of-the-art ultra-high throughput screening (uHTS) and high content screening (HCS) facilities and expertise
- Identifying Qualified Hits and Improved Hits for further optimisation into lead structures or research tools
- Generating and sharing knowledge with the broader drug discovery community on successful strategies in library design, assay development, screening, hit triaging, medicinal chemistry, and other early drug discovery techniques

“It was exciting to see competing pharma companies working together for a common goal to find new drugs for patients. The ELF partnership enabled companies like ours to share our compounds for high throughput screening in a pre-competitive space.”
Dr Daniel Basting,
ELF Project Lead and Senior Scientist at Bayer AG

Fast Facts
- Duration: January 2013 – November 2023
- Aim: To provide quality hit and lead compounds for innovative disease-related targets and phenotypes to accelerate the long, complex, and expensive process of drug discovery that is so critical to developing innovative medicines for global health challenges
- Total budget: €234 Million: 90 M (IMI from two rounds of funding); 134 M (pharma partners in-kind); 10 M (associated partners in-kind)
- Number of consortium partners: 33 participants: 13 academic + 10 SMEs + 9 pharmaceutical companies (EFPIA members) + 1 EFPIA associated partner (foundation/charity)
- Size of the ELF compound collection: 535,000 compounds (>350,000 EFPIA compounds, + ~190,000 compounds of the Public Compound Collection, developed by leading chemistry SMEs and academic groups)
- Screening infrastructure access for: Any researcher at any European SME or academic institution, provided that their screening proposal was selected by the ELF Review Committee
- Output of an individual screening campaign: A maximum of 50 qualified hit compounds, a subset of resynthesised hits, optimized hits through focused medicinal chemistry activities, and a data-rich report with information on experiments that were performed
- Geographical spread of the initiative:
o Consortium partners were spread over nine countries
o Public screening proposals were received from 20 countries: Norway, Sweden, Finland, Denmark, Ireland, UK, Netherlands, Germany, Poland, Belgium, Czech Republic, Switzerland, Austria, Hungary, France, Italy, Slovenia, Spain, Portugal, Israël
o Accepted proposals came from 17 countries: Norway, Sweden, Finland, Denmark, Ireland, UK, Netherlands, Germany, Poland, Belgium, Czech Republic, Austria, France, Italy, Spain, Portugal, Israël
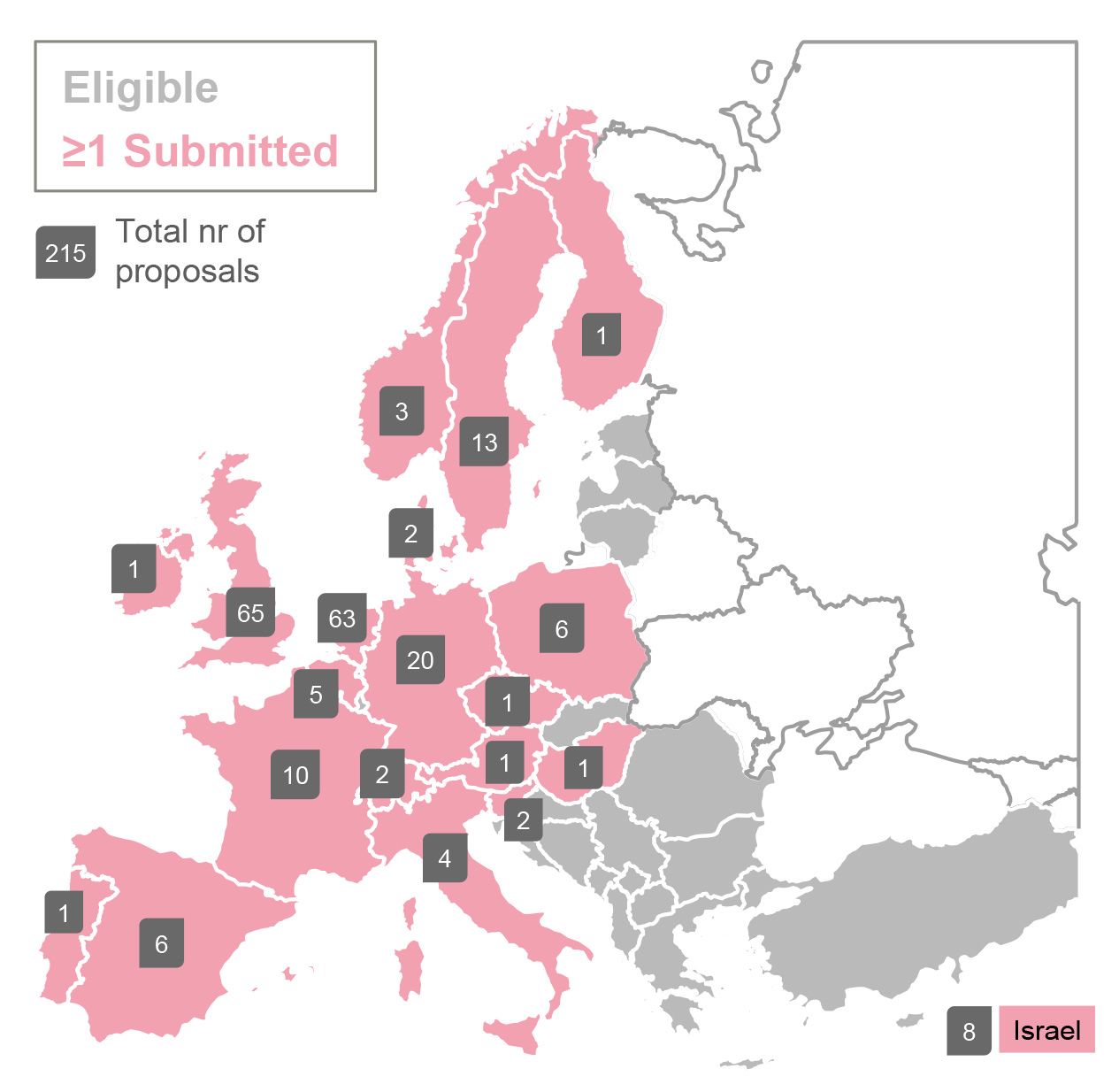
Submitted programmes per country.
Submitted programmes per country.
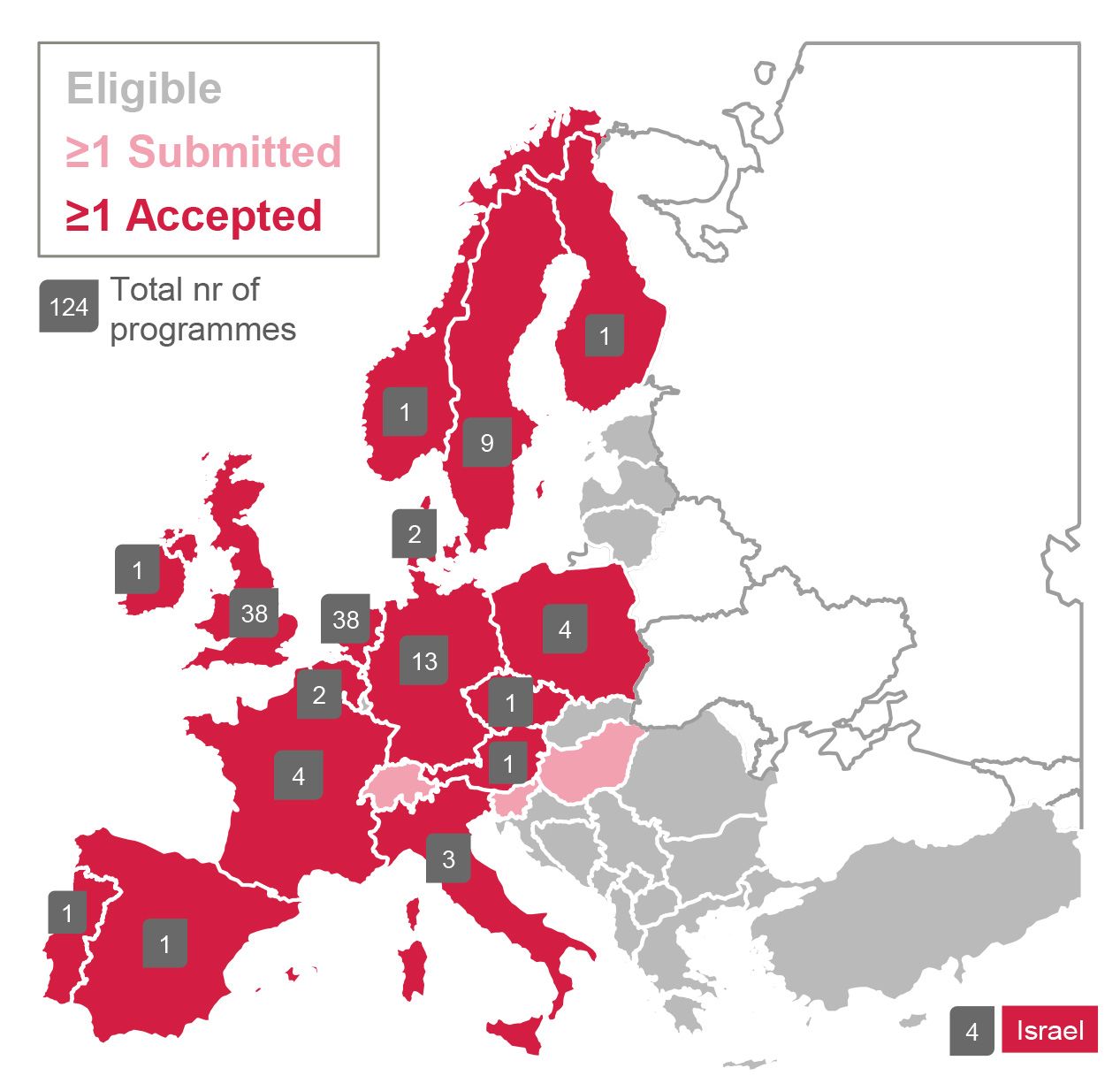
Accepted programmes per country.
Accepted programmes per country.

“A decade of the ELF has resulted in tangible assets and impactful output. With the contributions of many partners, we have successfully shown that collaboration with different organizations through a central core team can create waves and achieve results that translate into real impact.”
Dr Jon de Vlieger,
ELF Coordinator and Strategic Director at Lygature

Key Results 2013-2023
Assets, Infrastructure and Processes
- A High-quality compound collection of 535,000 compounds was established
- An industry-style screening platform was developed, which included assay development, ultra-high throughput and high content screening, hit selection, hit characterisation, hit resynthesis and confirmation, and hit to lead optimisation (of which the latter was only available during 2013-2018)
- A transparent proposal application and review process was developed, including a well-defined legal framework that balances the rights and obligations of all involved
- An award-winning software system was created (the Honest Data Broker) to enable screening data management and triaging, whilst ensuring confidentiality and patentability
- During the COVID-19 pandemic, proposals aimed to help tackle the pandemic were fast-tracked
- To support work at the ELF, assay development funds were established in Scotland and the Netherlands
Portfolio
- Over 300 drug discovery programmes were progressed at the ELF
- Of these, 209 were industry programmes, five were Medicines for Malaria Venture programmes, and 108 were crowdsourced programmes
- Screening proposals for crowdsourced programmes were received from 20 countries across Europe, indicating the richness and diversity of the ELF network
- A balanced portfolio of innovative drug targets and phenotypes in various disease areas (such as haematology, CNS & neurology, infectious diseases, inflammation and immunology, metabolic diseases, and oncology) was established. Target classes include enzymes, receptors, ion channels, protein-protein interactions, and various other targets and phenotypes
- In total, over 10,000 hit compounds were provided to programme owners for further hit optimization and drug development
Output
- 20 ELF programmes are available for partnering, and more will follow in the next years
- Work at the ELF has led to over 100 publications, with more expected to follow in the future
- Four start-up companies were initiated based on results coming out of the ELF, including Scandicure, Keapstone Therapeutics, and two companies from The Research Network (TRN)
- Nine patent applications were filed
- The ELF contributed to other European projects, such as IMI’s ENABLE, the Horizon Europe Project EXSCALATE4CoV, and the ISIDORe project
- Based on the ELF results, three Investigational New Drug (IND) applications were filed and are currently in clinical development
- ELF contributed to the education of over 100 European researchers on early drug discovery, for example via webinars and workshops
- 25 investigators and industry partners have provided testimonials on the impact of the ELF on their work
- 27 public programmes have elicited interest from the ELF’s industry partners. Outside of that, public programme owners and industry partners have collaborated to progress programmes
- As it typically takes about 7-10 years for targets and compounds to progress from identification to clinical testing, we expect more ELF programmes to progress to the clinic over the upcoming years
- The ELF model is truly unique and provides an example of pre-competitive, collaborative work. It can be seen as a blueprint for similar initiatives
A comprehensive overview of the impact of the ELF partnership was published in Nature Reviews Drug Discovery in 2021:
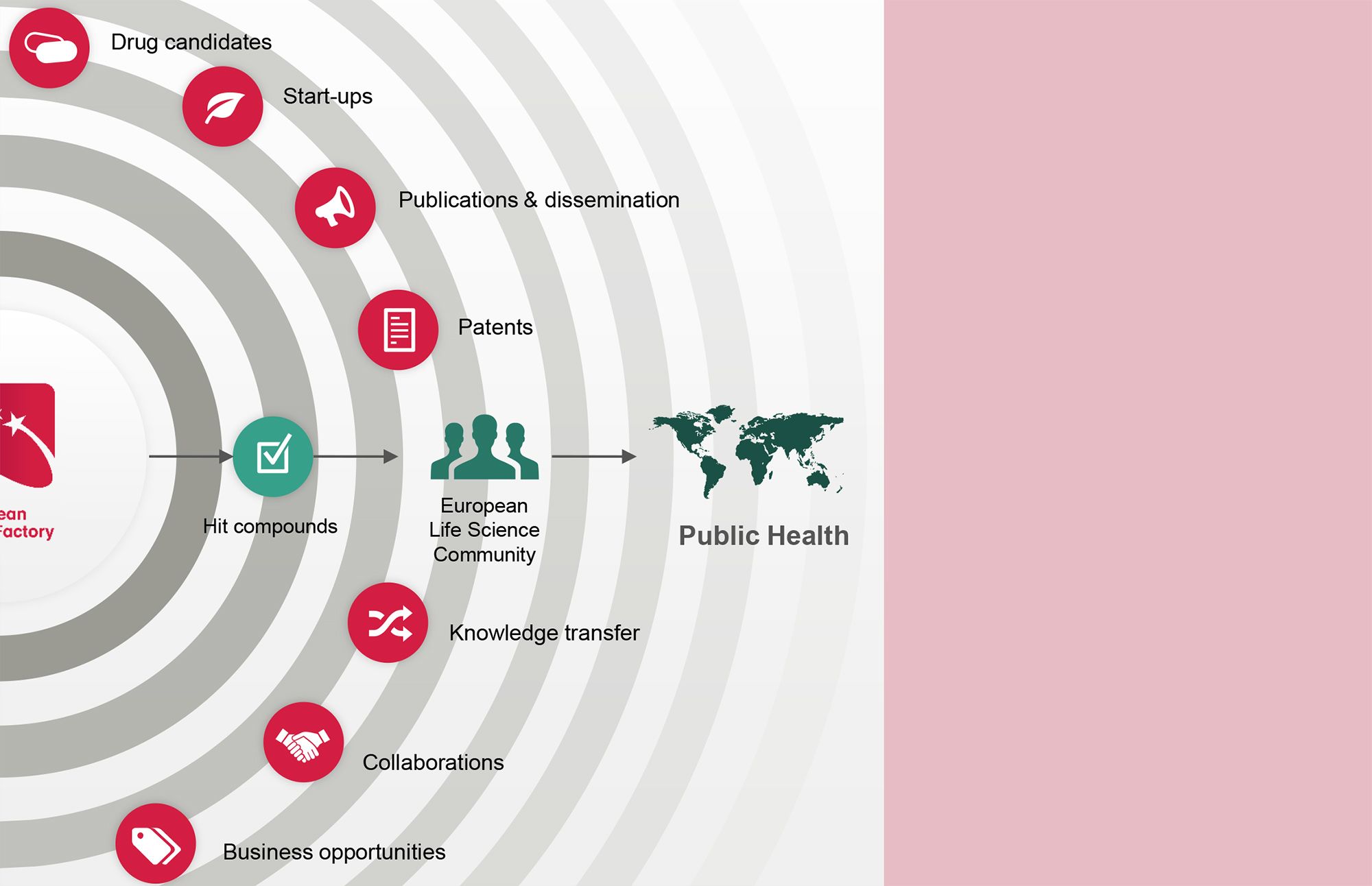
“The ELF has made a big impact across Europe supporting multiple project owners. It is the natural place to go to identify additional chemical matter. In that sense ELF generates starting points for drug discovery programmes and for further optimization towards drugs.”
Dr Phil Gribbon,
ELF Programme Owner and Head of Discovery Research at the Fraunhofer Institute of Translational Medicine and Pharmacology

Impact
The European Lead Factory has shown it can deliver health and wealth for society, make open innovation work, and boost science. The initiative successfully established a collaborative model for drug discovery that helped improve the efficiency and capacity of developing new medicines for unmet medical needs. It has demonstrated that it can deliver hit compounds that are highly suitable for further development, collective intelligence that capitalizes on previous investment, interesting partnering programmes, scalable (new) SMEs, and sustained corporate R&D in Europe. The ELF is an excellent example of collaborative open innovation with a focus on enhancing knowledge exchange between academia and industry.
Health and Wealth for Society
Using collective intelligence and capitalizing on previous public and private investments, the ELF combined the experience of big pharmaceutical companies with the agility of SMEs and the innovation of academia to unlock new medical innovations. Without the efforts of the consortium, many targets identified by academic researchers or SMEs would have gone unscreened and the high-quality chemical compounds in corporate libraries would have been underused (let alone expanded upon with novel compounds in a library open to all). Having tapped into this potential, the ELF increased the chance of finding relevant hits and leveraged earlier investments made through public funding of fundamental research and private investments in high-quality chemical matter. Ultimately, the ELF was able to unlock opportunities that no partner working alone would have been able to seize.
In addition to leveraging earlier investments in chemistry and biology, the activities of the ELF have had a positive economic benefit on the European research landscape. The collaboration produced investable programmes that led to hits or leads that could be progressed into clinical candidates. Through the partnership, academic groups, SMEs, corporations, and charities/foundations (e.g. the Medicines for Malaria Venture) were provided with the necessary tools and capabilities to develop their programmes outside of the ELF framework. This opened doors for new business ventures that could in turn attract funding and be scaled up into viable, growing European SMEs. The ELF has, without a doubt, helped strengthen the European research infrastructure and increase the competitiveness of its academic, SME, and industry partners.
Impact on Different Therapeutic Areas
Through its balanced portfolio, the ELF has delivered results in many different therapeutic areas, including those that pose some of the world’s biggest health challenges. Achievements within the different therapeutic areas range from the identification of tool compounds in diabetes and cardiovascular research to the announcement of collaborations between partners in metabolic diseases, and the launch of clinical studies in oncology (see below). As it typically takes about 7-10 years for targets and compounds to progress from identification to clinical testing, we expect more ELF programmes to progress to the clinic over the coming years. In addition to these successes, the partnership has contributed to other European projects, including IMI’s ENABLE and the Horizon Europe Project EXSCALATE4CoV. The following examples illustrate the impacts of the ELF drug discovery platform within various disease areas:
ONCOLOGY
Cancer is the second leading cause of death globally with rates continuing to rise. In 2022, new cancer cases in Europe rose by 2.3%, reaching 2.74 million. Providing new hope for the development of cancer drugs, the ELF partnership helped identify two drug candidates for cancer treatment that have progressed to phase 1 clinical trial testing.
ANTIMICROBIAL RESISTANCE
Referred to as the silent pandemic, antimicrobial resistance (AMR) is one of the greatest health threats that humanity faces today. The overuse and misuse of antibiotics are driving drug-resistant pathogens, leading to an increasingly urgent global health crisis. Research, based on the results and improved hit compounds delivered by the ELF, led to the discovery of a much-needed new class of drug that has the potential to reverse antibiotic resistance for conditions such as pneumonia and sepsis.
PANDEMIC PREPAREDNESS
During the first year of the COVID-19 pandemic, the ELF decided to prioritise COVID-19-related screening proposals through a fast-track procedure. One of the programmes selected for screening in 2020 progressed to hit-to-lead phase, leading to the discovery and development of novel antivirals against SARS-CoV2. Another programme on COVID-19 managed to successfully secure follow-up funding.
NEGLECTED TROPICAL DISEASES
Neglected tropical diseases (NTDs) threaten millions of people living in the poorest countries and communities around the world. As a project aimed at catalysing the discovery of new medicines for some of the world’s most complex health challenges, the ELF partnership made a concerted effort to attract more programmes addressing NTDs, achieving results in NTD drug discovery programmes. One example is the disease leishmaniasis, for which the ELF embarked on a screening programme. Another example is malaria through the ELF’s partnership with the Medicines for Malaria Venture (MMV), a partner of the ELF since 2018, which ran high-throughput screens for novel antimalarials.
THE CENTRAL NERVOUS SYSTEM (CNS) & NEUROLOGY
Within the field of CNS & Neurology, unmet medical need continues to be high. The ELF helped boost drug discovery in this area with successful developments for both chronic pain and anxiety disorders.

“The European Lead Factory can help researchers in any area find high-quality hits or leads. The results provided by the ELF are also very selective in terms of intellectual property, something that requires careful research. All of this provides a strong basis for future medicinal chemistry programmes.”
Maria Paola Costi,
Professor of Medicinal Chemistry at the Department of Life Science, University of Modena

The ELF Assets and Infrastructure
The ELF Compound Library
The success of High-Throughput or High Content Screening drug discovery campaigns depends highly on the quality of compounds in a screening collection. Over the course of the ELF, a high-quality compound library was established. The Joint European Compound Library (JECL) 2013-2018 was the beginning of the successful ELF collection. It contained a diverse range of high-quality compounds that were synthetically tractable and made excellent starting points for drug discovery projects. These compounds came from the proprietary collections of the seven founding pharmaceutical (EFPIA) partners of the ELF, and from the project’s own synthetic chemistry programme, leading to the establishment and availability of the ELF Public Compound Collection (PCC). The PCC successfully expanded chemical space to improve the discovery of novel chemical probes, novel chemical leads, and drug discovery efforts. Aspects that set the PCC apart from other libraries are an increased three-dimensionality, saturated ring systems, low Log-P, a large number of bases and few acids, novel cores, and an overall high chemical diversity. A full analysis of the newly designed compounds was performed by expert medicinal chemists from ELF partners Edelris and Symeres.
When the ELF received a second round of funding, the current library of approximately 535,000 compounds evolved from the JECL. By removing some compounds, and adding others from two new partners, Grünenthal and Servier, the collection was made even more robust. In a paper published in Drug Discovery Today, partners of the ELF detail the properties of the updated compound collection, showing that it is an attractive and highly diverse compound set that is complementary to commercial screening libraries. The fully updated collection was described in a paper by Van Vlijmen et al., 2021.
The ELF Screening Centre and Other Expertise
Another important ELF asset is its state-of-the-art screening centre, supported by different partners with different expertise. Together, they provided expert advice along the full screening journey, from assay development to Ultra High-Throughput Screening (uHTS) and High Content Screening (HCS), hit selection, hit characterisation, hit resynthesis and confirmation, and hit to lead optimisation (of which the latter was only available during 2013-2018).
Assay development and screening for uHTS programmes were performed at The Pivot Park Screening Centre (PPSC) located in Oss in the Netherlands. HCS took place at the National Phenotypic Screening Centre (NPSC) at the University of Dundee.
World-class compound logistics facilities of 1,100m2 were provided by BioAscent using their automated compound store in Newhouse, Scotland.
Hit characterization and hit-to-lead optimization were provided by BioAscent. Now part of BioAscent, the prior Newhouse group of the University of Dundee provided biological and biophysical data, medicinal chemistry, informatics analysis and modelling to the screening programmes of the ELF. The Biology, Chemistry and Medicinal Chemistry teams ensured that Qualified Hit Lists (QHLs) and Improved Hit Lists were of the highest quality and value to programme owners, with tailored activities for each programme. Pharmaceutical industry-standard facilities combined with some of the latest technologies and expertise supported hit identification and follow-up with biochemical and biophysical data, and in collaboration with ELF’s SME partners Edelris, Symeres, Sygnature Discovery and Taros, resynthesis and hit confirmation data post-QHL.
Protein production was provided by the Biotechnology group of the Structural Genomics Consortium (SGC) at the University of Oxford.
Find out more about ELF's state-of-the-art drug discovery facilities and dedicated expert personnel on our website:
Read an interview with the ELF programme clearance team (PCT) from Bioascent and PPSC about selecting the best and most interesting compounds for ELF programme owners:
Honest Data Broker
An award-winning software system, the Honest Data Broker (HDB), was developed to enable screening data management and triaging, whilst ensuring confidentiality and patentability. The HDB, a bespoke high-throughput screening triage and project management application, was developed to support the unique scientific and intellectual property (IP) requirements of the ELF. It enabled scientists to select the best compounds emerging from the screens while ensuring that all activities remained within the IP framework. The HDB was hosted on Biovia's ScienceCloud and comprised a full suite of cheminformatic tools to enable filtering, clustering, similarity searches, activity modelling, multiparameter optimisation and is seamlessly linked to established visualisation tools on the same cloud platform.
The Screening Proposal Process
To ensure that researchers had a good understanding of what the application process entails, and how their screening proposals would be reviewed, a transparent proposal application and review process was developed. In addition, a well-defined legal framework to balance the rights and obligations of all involved was implemented. Throughout the application process, researchers also received scientific advice on their appication with which they could further improve their proposal. As a result, excellent proposals across several different therapeutic areas were received. Investigators frequently mention that they benefitted from the expert knowledge available at the ELF, both during the application process as well as during the programme execution. This helped deepen their understanding of overall early drug discovery.
This interview provides more insight into the screening proposal and selection process:
SMEs as an Important Driver for Success
Public–private partnerships are, at the onset, often accused of a certain degree of inertia that results from a complex partner mix, opposing interests, democratic decision-making, and high demand of transparency. The ELF circumvented this and managed to be highly effective and fully operational within a year through a clear governance structure, intellectual property (IP) regulation, IT software solutions, in combination with the drive of the ELF partners to deliver valuable output for the benefit of the wider community. The collaboration worked far beyond the objectives of any single partner.
Certainly, the high ratio of SME involvement in the ELF had a pivotal role in accelerating many processes. SMEs have over the past decade worked closely with and for the larger pharmaceutical industry, as well as with public research institutes in early drug discovery projects. The ELF provided the SMEs with a showground to further prove their abilities. The participating SMEs were instrumental in implementing the production and management of the compound library and performing the screening. Their agility and emphasis on producing results had a professional influence on the ELF machinery, making it the well-oiled initiative it became.
Consortium coordination and communication
Designed as a true public-private partnership, the ELF governance reflected the interests of all partners involved. The ELF Project Executive team consisted of three members from the Project Lead Bayer AG, the ELF Head of Screening/Coordinator from Lygature, and the ELF Head of Chemistry from the Chemistry SMEs.
The Project Executive team was supported by a dedicated Programme Office, managed by Foundation Lygature, an organisation with an impressive track record in setting up and managing public-private partnerships. Lygature was also responsible for the coordination of the review and selection of proposals for screening programmes, related portfolio management, assembling the final reports for programme owners, and acted as the central point of contact for potential applicants and programme owners. In addition, Lygature played an essential role in safeguarding the confidentiality of the screening programmes both inside and outside the HDB IT-system (see above). It was also responsible for communication of the consortium activities to the scientific community, including management of the website and social media platforms, creation of all consortium materials, and organization and support of webinars and other meetings.
This interview provides more information on the role of the ELF Programme Office team at Lygature:



“The opportunity to partner with serious and accomplished drug development efforts was a critical feature of the European Lead Factory.”
Professor Jason Swedlow,
ELF Consortium Member and Head of the National Phenotypic Screening Centre at the University of Dundee

Portfolio
From the start, the ELF set out to recruit the most innovative, high-quality programmes from across Europe in all disease areas. The partnership combined complementary industry and crowdsourced portfolios to address critical unmet medical needs. For the crowdsourced portfolio, an application and review process was established to ensure scientific quality, novelty, and (technical) compatibility with the available screening infrastructure. The ELF took a strategic approach to its portfolio management aiming for a balanced portfolio of projects that were selected based first and foremost on patient benefit, but also on scientific impact, risk diversity, and economic potential of the full portfolio.
Overall, more than 300 drug discovery programmes were progressed through the ELF over the course of 11 years. Of these, 209 were industry programmes, five were programmes for the Medicines for Malaria Venture, and 108 were crowdsourced programmes. The crowdsourced programmes were selected from 215 submitted proposals. Screening proposals for crowdsourced programmes were received from 20 countries across Europe, indicating the richness and diversity of the ELF network. Approximately 30% of the crowdsourced proposals were submitted by SMEs.
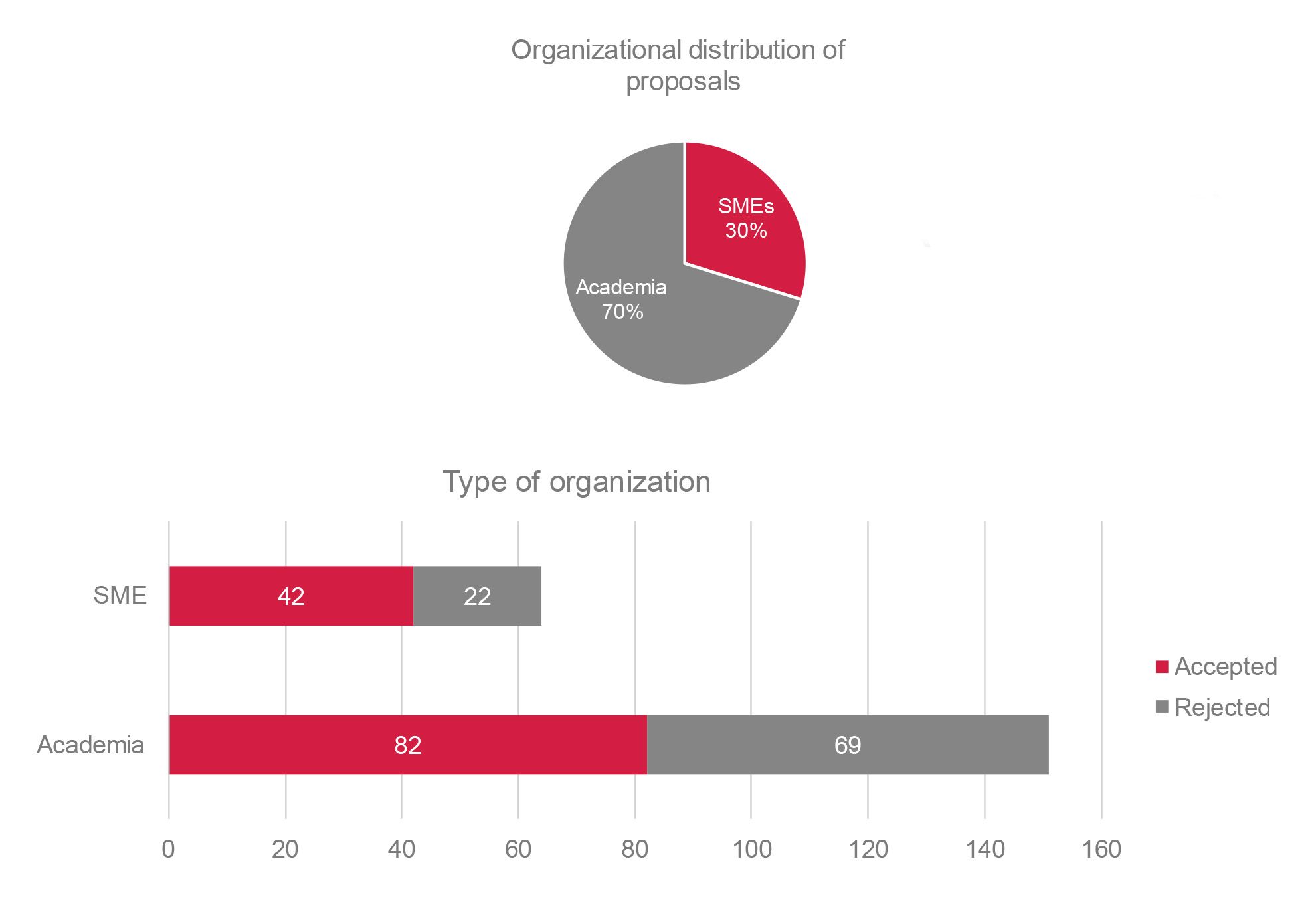
SMEs submitted 30% of all crowdsourced proposals (2013-2022).
SMEs submitted 30% of all crowdsourced proposals (2013-2022).
A balanced portfolio of innovative targets and phenotypes in various disease areas was established. The ELF contributed to developments for cardiovascular diseases & haematology, CNS & neurology, infectious diseases (including neglected tropical diseases and COVID-19), inflammation and immunology, metabolic diseases, and oncology.
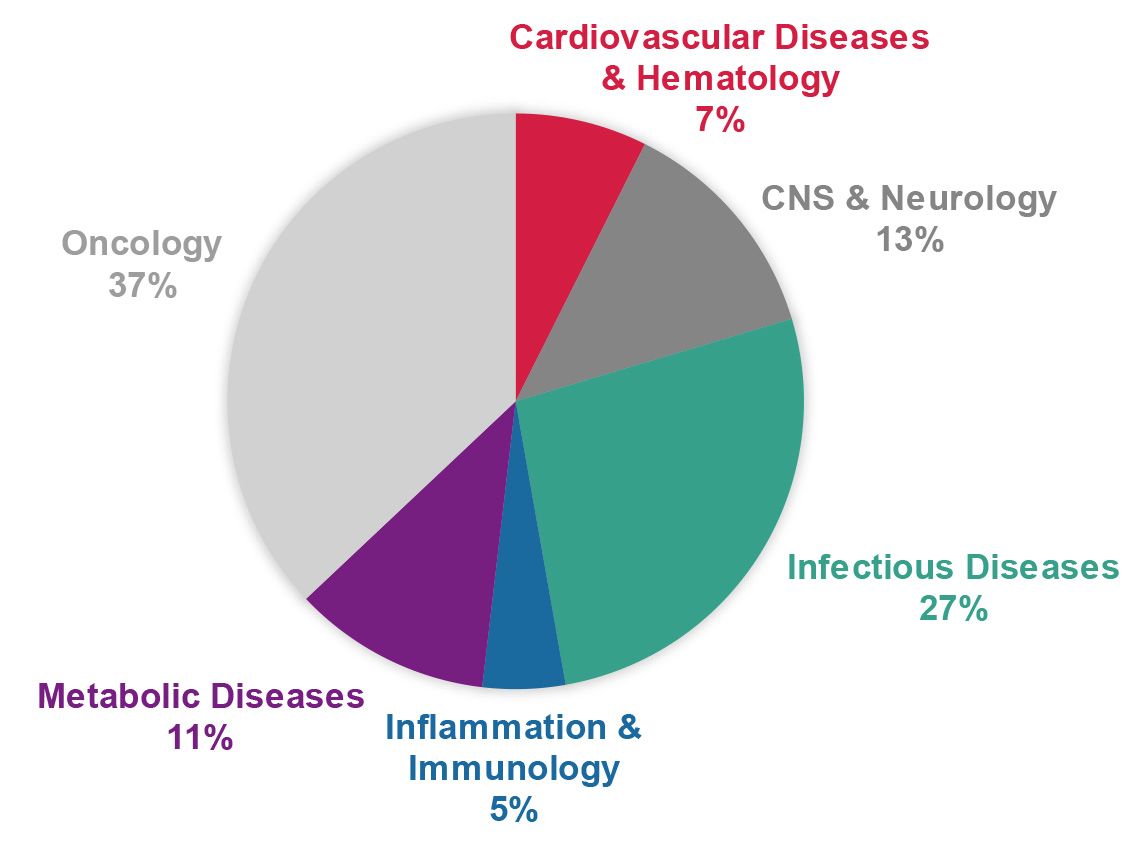
Crowdsourced programmes.
Crowdsourced programmes.
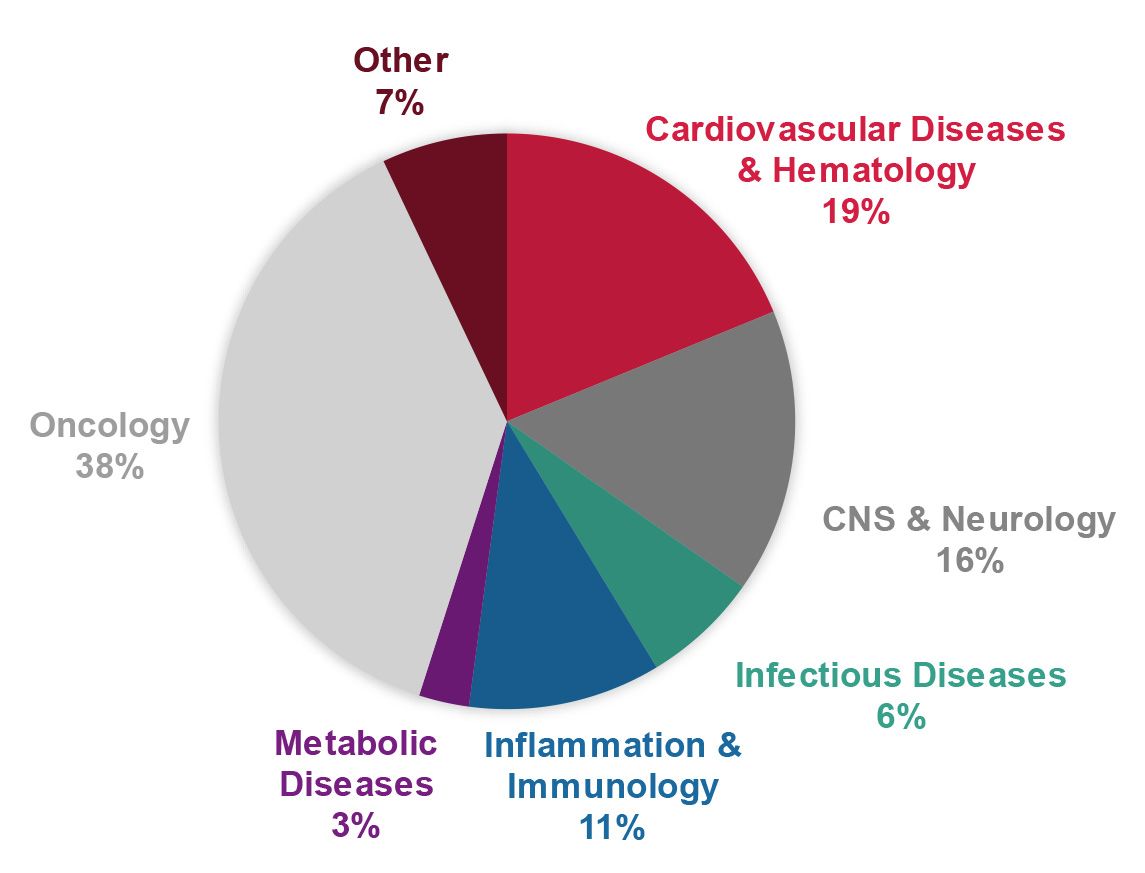
EFPIA programmes.
EFPIA programmes.
Target classes include enzymes, receptors, ion channels, protein-protein interactions, and various other targets and phenotypes.
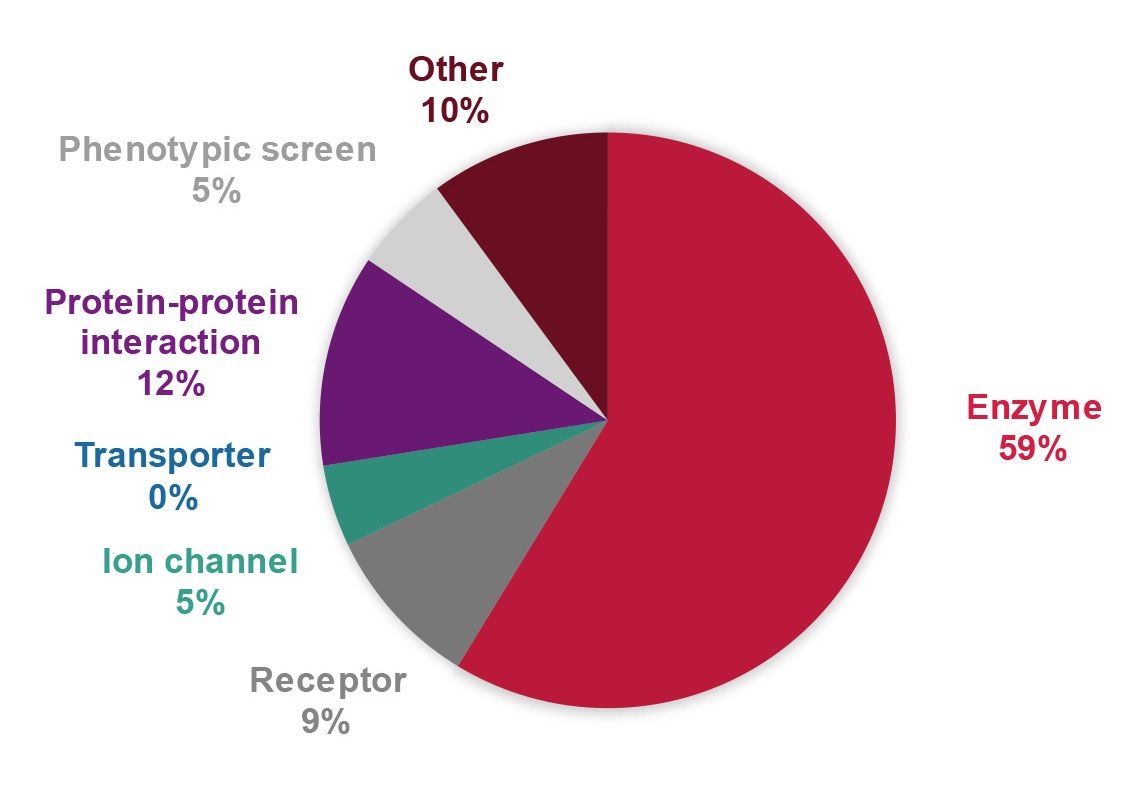
Crowdsourced programmes ELF total.
Crowdsourced programmes ELF total.

EFPIA programmes.
EFPIA programmes.
In total, over 10,000 hit compounds were provided to programme owners for further hit optimization and drug development.

“The European Lead Factory provided us with exciting opportunities for the advancement of anti-malarial drug discovery.”
Dr James Duffy,
ELF Consortium Member and Director of Drug Discovery at the Medicines for Malaria Venture

Efforts to Continue the Success Story
The decade-long success story of the ELF would not have been possible without the support of the Innovative Medicines Initiative (IMI). The IMI funded the ELF with €196.5 million for the initial five years. Based on the success achieved under this first project term, it awarded the consortium a further €36.7 million.
The second round of IMI funding ran from 2018-2023, under the banner ‘ESCulab’ (European Screening Centre: unique library for attractive biology). Whilst the first phase of the ELF focused on defined biochemical targets, the second phase broadened this scope to target-agnostic phenotypic screens and introduced high-content screening to further increase the range of therapeutic applications.
During the second project term, the charity Medicines for Malaria Venture (MMV) joined the consortium to guarantee access to the ELF’s screening capabilities for multiple programmes. As the ELF’s first product development partnership, it was hoped that this collaboration would serve as an example of the benefit of combining the considerable scientific expertise of researchers in the charity sector with the ELF’s resources to expedite drug development for neglected diseases and other non-commercial indications. In particular, the ELF provided charities such as MMV with access to a high-quality, novel, drug-like screening library that they would otherwise not have had access to.
Establishing a long-lasting drug discovery platform was an important aim of the initiative from the beginning. Throughout, the partnership focused its efforts on the continuation of the ELF, actively exploring several avenues to maintain the ELF compound library beyond the lifetime of the project. One of the most notable examples of this was the establishment of a well-defined framework to engage, outside the scope of the IMI project, with charities and non-profits around the world. In early 2022, the consortium launched a new partnering initiative for charities and foundations. The aim here was to provide these organisations, within and outside of Europe, with access to the ELF’s well-established assets, services, and network of experts to support them in their mission of delivering new therapies to patients. With this step, the ELF sought to make a worldwide impact in various areas of unmet medical need, and across all disease areas and geographies. Linked to this work, a Dutch assay development fund was launched to support scientists in the Netherlands working with a charity or foundation on an early drug discovery project.
The partnering programme for charities and foundations was an ambitious and complex initiative to implement. After thorough investigation and the exploration of multiple avenues, it became apparent that the charity/non-profit scenario would require another tier of co-funding to allow the ELF to continue and take on its semi-public role. Co-funding was not found amongst the stakeholders and it therefore became clear that the ELF’s activities would have to wind down at the end of the second project term.
Over the years, the consortium has worked hard with relevant stakeholders to consolidate the ELF’s position in the pre-competitive drug discovery landscape. A significant effect of these sustainability efforts is the impact of one of the main deliverables of the initiative: the Qualified Hit Lists. These continue to become visible as the first clinical candidates from early programmes are now being tested in clinical studies.
Throughout its decade-long journey, the consortium made it a priority to meet the needs of its stakeholders and worked hard to execute a sustainability strategy that would keep it sufficiently funded. The motivation always remained to serve the drug discovery community at large and take on a semi-public role in finding starting points for new medicines in therapeutic areas of high public need.
A key success factor of the ELF has, and will always be, the fact that the individual partners, whose commercially successful and sometimes competing businesses, were able to work together under the joint ELF mission of delivering innovative drug discovery starting points that would make a tangible contribution to enriching the European research landscape.
The ELF’s legacy and the impacts of its success will continue to be driven by the progression of individual drug discovery campaigns towards the clinic.

“Translational research needs funders to support the early and obviously more risky steps in drug discovery. Without that support, we'll never get the exciting basic science through to the clinic.”
Professor Sara Brown,
ELF Programme Owner and Clinical Academic Dermatologist at the University of Edinburgh

Wrapping up a Successful Partnership and the Legacy of the ELF
As eluded to above, over the course of the second project term, the priorities and remits of several partners changed, as did the world of drug discovery and funding opportunities. As such, the possibility for the continuation of compound sharing within the ELF without public-private co-funding became a reality that was no longer achievable. With this, after a decade of fruitful collaboration, the ELF activities have wound down and the initiative officially comes to a close at the end of 2023.
The partnership is now committed to finalising the remaining programmes and providing the last Programme Owners (investigators) with the best possible start for their drug discovery projects.
The results of 11 years of the European Lead Factory have shown that the initiative has greatly contributed to the advancement of new therapeutic discoveries and overall education about early drug discovery. These results have benefitted academia, SMEs, larger industry, and society as a whole, creating long-lasting, real-world impact for many different disease areas, including those of high unmet medical need.
The project has been a true example of successful public-private collaboration. The impacts of the ELF will certainly continue to become apparent over the coming years. Everyone involved looks back on a successful, long-running partnership with a wealth of knowledge gained and a host of tangible results to be proud of.

The final consortium meeting, November 2023.
The final consortium meeting, November 2023.

“The notion of 'sharing' persists to this day, and academia and EFPIA continue to adopt an open-minded approach that welcomes new ideas and methodologies. The broad involvement of third parties utilizing the ELF infrastructure to advance their scientific research has shifted mindsets in the community and is poised to endure over time.”
Dr Dirk Finsinger,
ELF Consortium Member and Director in Medicinal Chemistry at Merck


Academic partners.
Academic partners.

EFPIA & associated partners.
EFPIA & associated partners.

SME partners.
SME partners.

The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement No 115489, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7 / 2007-2013) and EFPIA companies’ in-kind contribution.
This project has also received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 806948: ‘ESCulab: European Screening Centre; Unique Library for Attractive Biology’. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA and Medicines for Malaria Venture.
Want to learn more about us?
© Created in November 2023 by the ELF consortium and Foundation Lygature. All rights reserved.